Success in Development of Novel Chirality Sensing Technique Enabling Easy Determination of Optical Purity
High Potential as a Simple Technique for Ensuring Safety in Synthesis of Pharmaceuticals
2013.07.17
(2013.08.23 Update)
National Institute for Materials Science
A research group including Dr. Jan Labuta, a MANA Research Associate, and Dr. Jonathan P. Hill, a MANA Scientist, both of the NIMS International Center for Materials Nanoarchitectonics (MANA), succeeded in developing a novel technique for simple measurement of chirality and optical purity.
Abstract
- A research group led by Dr. Jan Labuta, a MANA Research Associate, and Dr. Jonathan P. Hill, a MANA Scientist, who are both members of the Supermolecules Unit (Unit Director: Katsuhiko Ariga) of the International Center for Materials Nanoarchitectonics (MANA; Director-General: Masakazu Aono), National Institute for Materials Science (President: Sukekatsu Ushioda), in joint work with Dr. Shinsuke Ishihara, who is a ICYS-MANA Fellow at the NIMS International Center for Young Scientists (ICYS; Managing Director: Kenjiro Miyano), and researchers at Charles University in Prague (Univerzita Karlova v Praze, Czech Republic) and others succeeded in developing a novel technique for simple measurement of chirality and optical purity. This technique offers excellent simplicity and practicality, and its high versatility has also been demonstrated.
- Certain molecules have a property of mirror symmetry, or "chirality," which means that molecules with different chirality are mirror images of each other. That is, although they have the same shape, like a person's right and left hands, those shapes cannot be superimposed on each other. (In fact, chiral molecules are frequently called "right-handed" or "left-handed.") The properties and bioactivity of chiral organic molecules and biomolecules are completely different, even though the molecules have identical chemical formulas. For example, while the left-handed variant of the molecule called bupivacaine is an effective painkiller, the right-handed variant displays cardiotoxicity. As this example suggests, chirality sensing techniques which can distinguish right-handed and left-handed chirality and determine their optical purity are critically important.
- Optical purity is one key parameter of chiral molecules, and is expresses the ratio of right-handed and left-handed molecules. In particular, in drug manufacturing, in addition to quality control of medical products, it is also important to determine their optical purity in each stage of synthesis and optimize the manufacturing process. For this reason, the development of a simple, inexpensive technique has been demanded.
- In the present research, the MANA-led team succeeded in developing a novel technique for determining optical purity by utilizing nuclear magnetic resonance (NMR) and a new symmetrically-structured porphyrin resolving agent which was developed independently by the team. The key feature of this research is the use of the symmetrical porphyrin resolving agent, which does not have a chiral structure. This means that the molecules do not form structural isomers (diastereomers) even when they bind with a chiral object of measurement (e.g., a pharmaceutical molecule). This distinctive feature clearly distinguishes the present research from similar conventional research. The mechanism of this technique is based on the breakdown of the structural symmetry of the porphyrin resolving agent by the bonding of chiral molecules, which was elucidated by binding equilibrium model equations, quantum mechanics calculations, and molecular dynamics simulation. The developed symmetrical achiral porphyrin resolving agent offers high versatility as well as universality enabling measurement of the optical purity of diverse species of chiral molecules, such as chiral carboxylic acids, esters, protected amino acids, ketones, alcohols, and others.
- Because this method provides a quick, simple tool for determination of optical purity, use in the drug manufacturing industry is expected, among others. Since the newly-developed technique uses absolutely no chiral elements in either the measurement method or the resolving agent, it is expected to be suitable for real-time analysis in asymmetric synthesis, chiral amplification reactions, and similar applications. With conventional techniques, adverse effects on asymmetric synthesis, chiral amplification reactions, etc. had been a concern.
- To distinguish the novel chirality sensing molecule developed in this work, which is an achiral molecule, from conventional resolving agents consisting of chiral molecules (NMR chiral shift agents), the new chirality sensing agent was named "prochiral NMR solvating agent (pro-CSA)."
- This research achievement will be announced in the online edition of the English scientific journal "Nature Communications" at 18:00 July 17, 2013 Japanese time (10:00 July 17 local time).
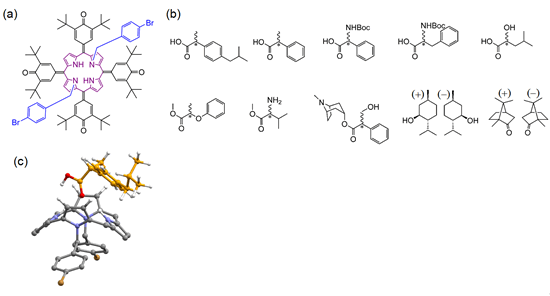
Figure 1. (a) New symmetrical achiral porphyrin resolving agent, which is an independently-developed NMR prochiral solvating agent (pro-CSA), (b) examples of the diverse chiral molecules whose optical purity can be obtained using pro-CSA, and (c) a schematic diagram of a 1 : 1 complex consisting of a porphyrin derivative and a chiral molecule.
